About the User Resource
General Description of the User Resource
The User Resource is a U.S. National User Facility Resource and is located at the Department of Energy’s Lawrence Livermore National Laboratory (LLNL), within the Center for Accelerator Mass Spectrometry (CAMS) and the Physical and Life Sciences Directorate.
The User Resource for Biological Accelerator Mass Spectrometry (AMS) has been established to make AMS available to researchers who have a need for accurately measuring very low levels of radioisotopes in their research. This User Resource is the only one of its type and is continually focusing on methodological and technical refinements to improve the efficiency of operation. These enhancements result in more rapid and easier sample analysis for our users, as well as the capability to expand the number of user projects and users.
The User Resource’s forte is its ultra-high sensitivity quantitation of radiocarbon and selected other radioisotopes for research studies where isotopes are required. AMS is a specialized and unique type of mass spectrometric method for quantifying extremely low concentrations of long-lived radioisotopes, such as the commonly used biochemical tracer 14C. AMS can measure attomoles of radiocarbon with a precision of better than 3%. This corresponds to the need for less than 0.1 DPM-equivalent of labeled agent per gram of biological sample. AMS has use when sample is limiting, specific activity is very low, when the level of isotope that can be used is very limited (human studies) or when trying to study events that occur with very low frequency or at very low concentration.
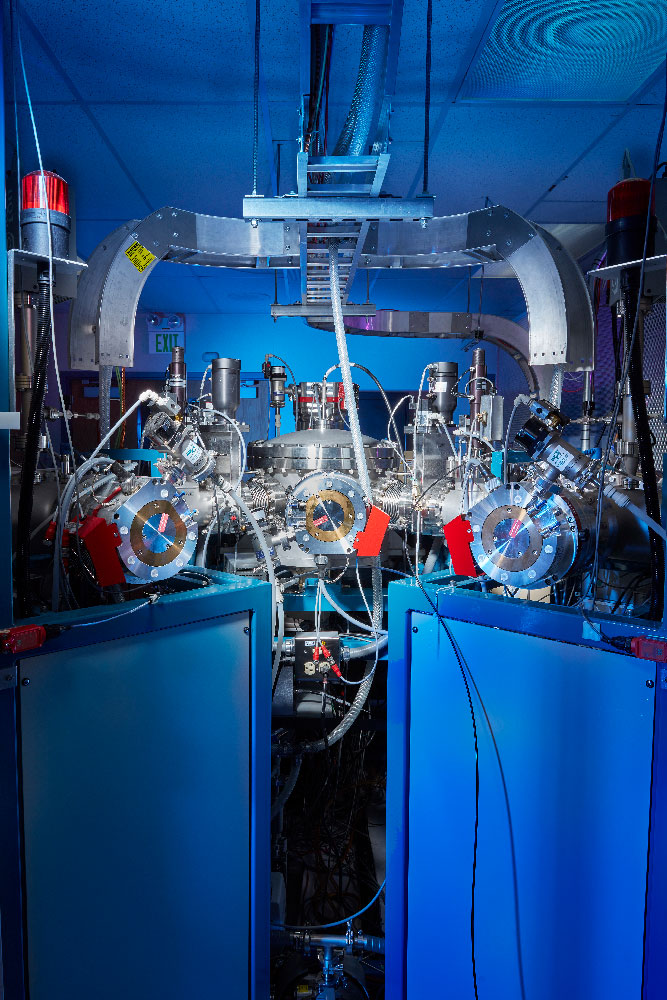
The centerpiece of the User Resource is a dedicated biological AMS spectrometer in a room configured with biosafety level 2 controls. This spectrometer is a 250kV Single Stage Accelerator Mass Spectrometer (SSAMS). This spectrometer is equipped with two hybrid ion sources, each capable of accepting samples as either solid graphite or gaseous CO2. In addition, the User Resource also has access to a 10-MV AMS spectrometer used for high-precision, bomb-pulse dating research.
Resource Major Objectives
1. Improve the efficiency of operations for AMS measurements through procurement and installation of new interfaces, ion source improvement, as well as upgrades to our data analysis codes.
2. Increase accessibility and visibility of ultra-sensitive 14C measurements for the biomedical research community by training new investigators and expanding our National User base.
3. Provide high throughput, ultra-sensitive 14C analysis for the NIGMS and NIH use community.
Resource Operating Procedure Summary
The National User Resource is operated under the general guidelines of the National Institutes of Health (NIH), National Institute of General Medical Sciences (NIGMS), and the recommendations of a National Advisory Committee. Standardized procedures have been developed for biomedical researchers to obtain access to the User Resource. This process entails each potential user submit a proposal or letter of intent regarding their scientific problem which is peer reviewed for acceptability. Projects approved for incorporation in the User Resource will be serviced as time, schedules, and resources permit. The User Resource enables NIH-funded and other biomedical investigators no cost access for biological AMS analysis.
Resource Scientific Mission
The User Resource for Biomedical AMS was established to apply the technology of AMS in broad-based biomedical research. The User Resource’s niche is to fill the need for ultra-high sensitivity quantitation with very small samples or with very low isotope concentrations. It is the only instrumental method capable of quantifying radioisotope-labeled agents routinely in real-world samples with such precision and sensitivity. The most significant work enabled by AMS is likely to be quantification of metabolic pathways in human health states and comparison to ex vivo and in vivo animal models.
Because this User Resource is presently the only of its kind in the country, we believe LLNL will continue to be the primary facility providing AMS capabilities in high enough capacity to serve the biomedical science community. We believe the technology made available as an NIGMS National and Regional Resource will enable a deeper understanding of the etiology of human health concerns by (1) enabling the quantification of pharmacokinetics and other molecular endpoints directly in humans; (2) offering the ability to conduct quantitative studies using biologics such as proteins or lipids, and thereby reducing the amount of radioisotope usage in biomedical labs; and (3) enabling more relevant studies of metabolic pathways in health and disease through the use of much lower, more biologically-relevant, concentrations of metabolic substrates in cells and intact organisms. These studies are already underway in the fields of toxicology, pharmacology, immunology, cell biology, cancer biology, and systems biology.
The development of liquid sample AMS is allowing us to meet the needs of User Resource users by providing a service resulting in more rapid and easier sample analysis. The spectrometer is equipped with two hybrid ion sources, each which is capable of accepting samples as gaseous CO2. Samples may be presented to the interface as either discrete liquid drops or through the continuous direct output of a Waters Acquity H-Class HPLC. Flow from the HPLC may be quantitatively split between the moving wire-AMS interface or to a coupled Waters Xevo G2-XS quadrupole-time-of-flight mass (QTOF) spectrometer. This enables accurate mass analyses for a variety of analytical applications, including metabolite profiling, identification, characterization, and quantification of both small and macromolecules with mass to charge ratios (m/z) ranging from approximately 50–20,000. We have established a 200 zmol 14C and 40 ng C lower limit of quantitation (LLOQ) with an upper limit of 50 amol 14C and 2 μg C in a single drop. We have used these interfaces to provide measurements for a number of studies that require metabolite separations using HPLC, DNA-adduct measurements, and quantification of drug incorporation into single cells.









